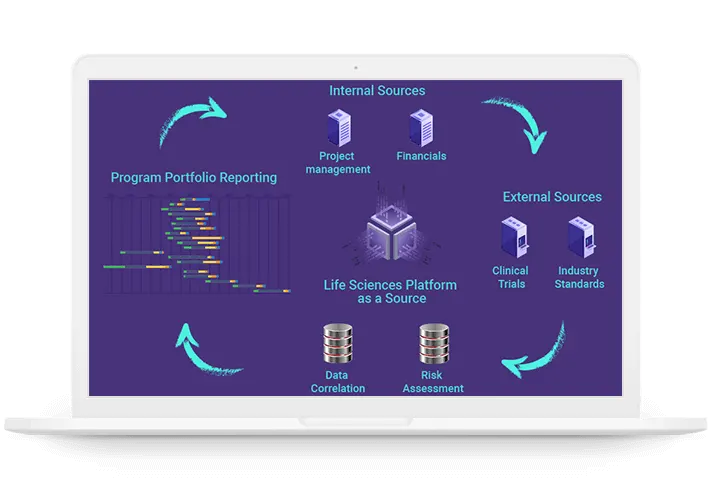
Single source of truth
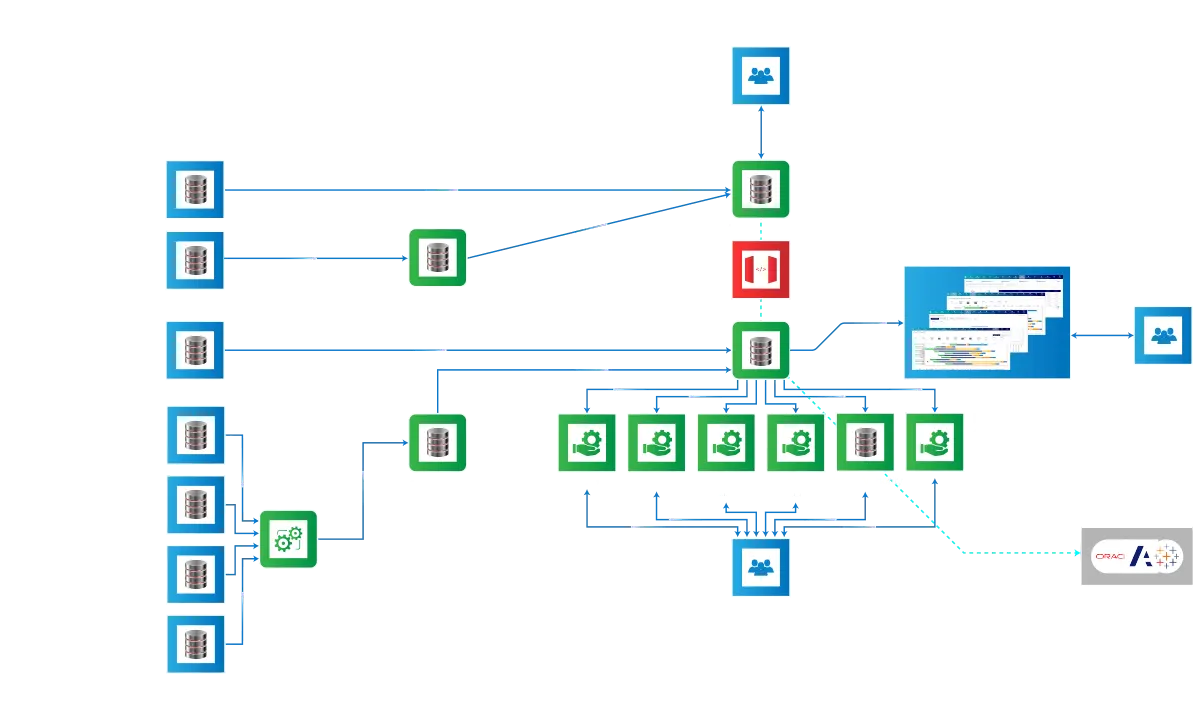
An star schema data warehouse is at the base of the platform
Program management, clinical trials, human resource and financial data are all
correlated and integrated in one database
Likelihood of approval, probability of running and other value added information is
input through functionality available in the platform
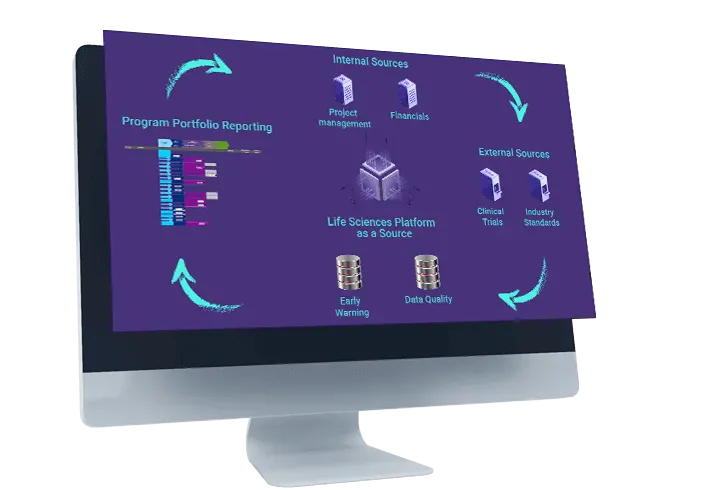
Risk, Data Quality and Early Warning
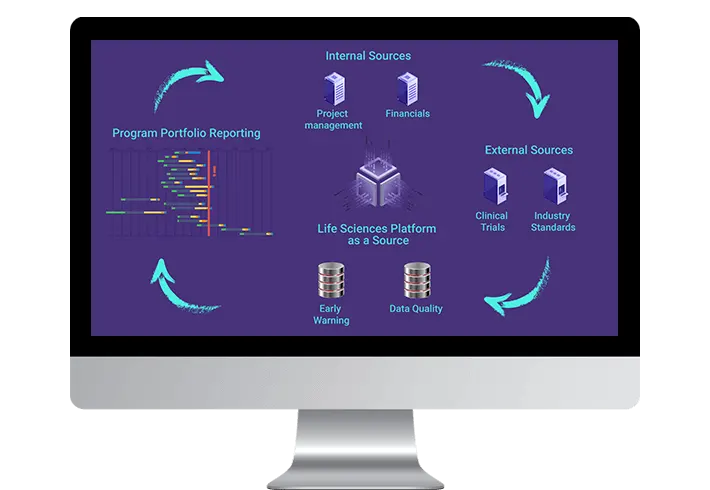
Early warning system alerts to missed or likely to be missed required milestones
providing visibility to projects at risk within the portfolio
Early warning system allows for timely corrective actions avoiding costly FDA
denials
Early warning system captures delays in patient recruitment and abandonment rate giving
protocol leaders visibility into potential study delays
Early warning system provides classification functionality thus enabling root cause
analysis and elimination of factors that cause delays and abandonment
Product pipeline analysis and reporting
Future view of risk adjusted drug development portfolio across geographies, therapeutic
areas, diseases, indications, etc. for all clinical milestones
Forecast of regulatory milestones and stage gates by geography, therapeutic area,
disease, indication, etc.
Current state of drug development portfolio, rate of patient recruitment, by study site
and study phase as well as by therapeutic area, disease and indication
Current portfolio performance relative to targeted milestones and submissions
Production planning and scheduling
Production planning based on forecasted clinical trial milestones, patient acquisition
rate and total number of patients
Integration of production schedules with budget and resource utilization information to
provide a holistic view of production pipeline across compounds
Synchronization between clinical trials and production of drug substance, delivery
mechanism and dosage is all accomplished within a single analytical platform
Tight integration with source systems ensures real time analysis and timely
decisions



